Biology and therapeutic value of T cell receptors

Busch group, May 2023
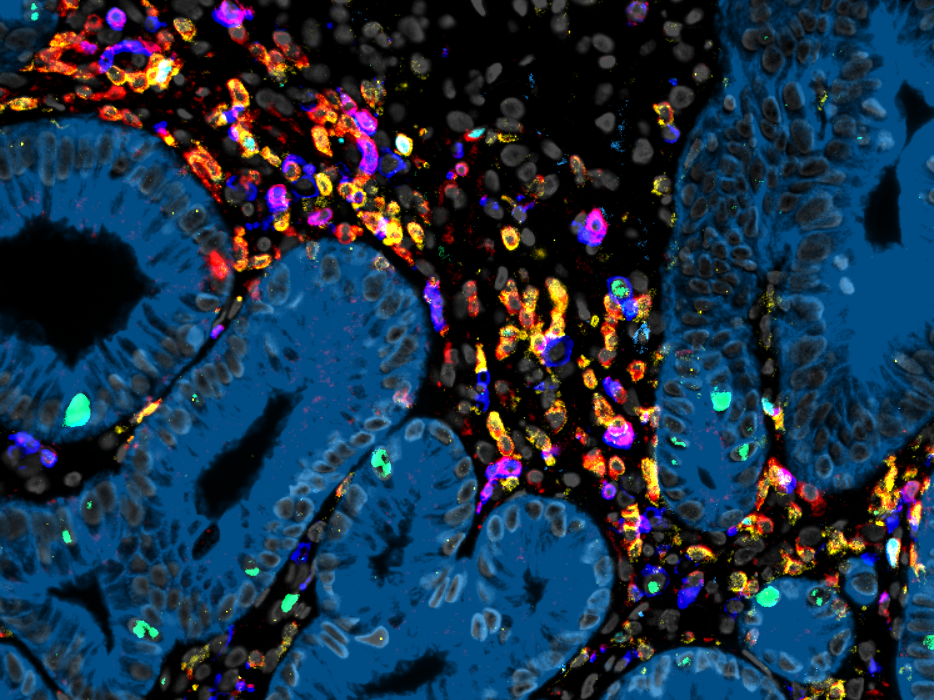
The T cell receptor (TCR) serves as the unique fingerprint of a T cell, dictating its target specificity, fate, and functions. T cells employ the TCR to discern between infected or tumor cells and healthy cells. This specific recognition, in turn, triggers effector functions, leading to the elimination of abnormal cells or the support of other immune cells. Our research group is primarily dedicated to unraveling the intricate workings of the TCR, particularly its "avidity" towards its targets, as it fundamentally influences the characteristics of T cells. Concurrently, we aim to apply this knowledge in developing increasingly potent cell therapies using TCR-engineered T cells for combating infectious diseases and cancer.
*dieser Inhalt ist nur auf Englisch verfügbar*
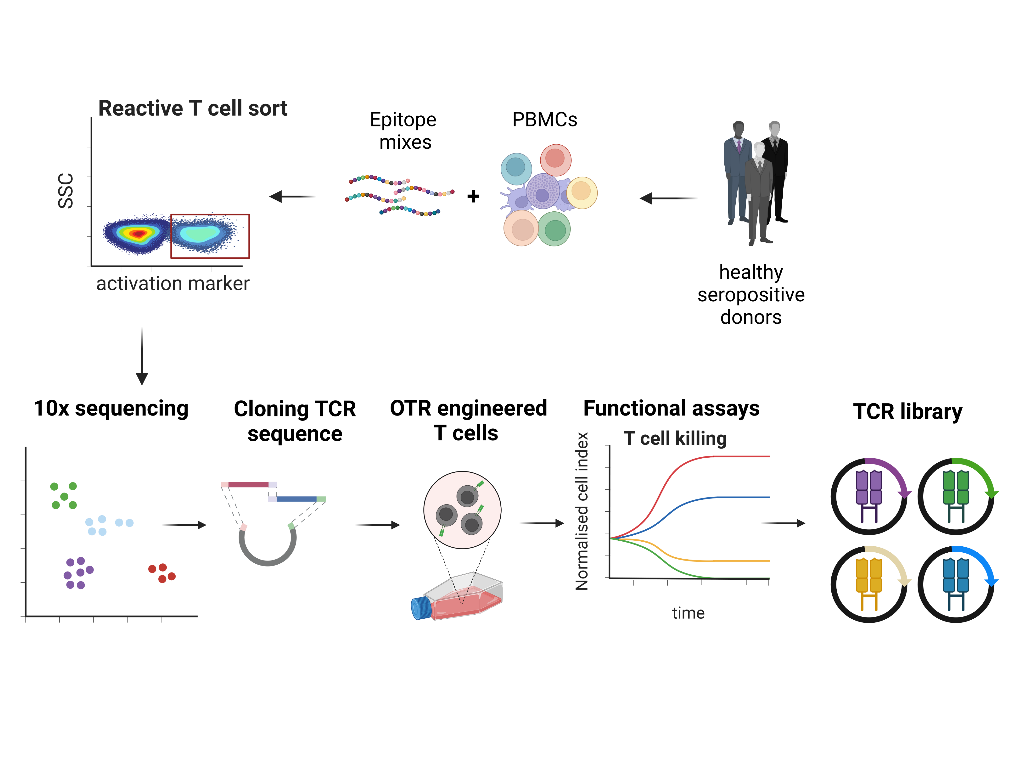
TCR libraries for infection diseases and cancer
T cells evolved over millions of years to protect the host from infections through the specific recognition and efficient killing of diseased cells. This concept has been widely used thera-peutically over the last decades to treat infections and tumors via the adoptive transfer of naturally occurring, antigen-specific T cells. Recently, the engineering of T cells with antigen-specific TCRs has offered the possibility to generate more reliable and versatile “living drugs”…
It's a matter of affinity
TCR affinity is a quantitative measurement of the binding strength of the TCR to its target. So far, it has been shown that TCR affinity determines the fate and efficacy of T cells in immune responses. However, effective TCR affinity for cellular therapy is also dependent on the disease context and differs between autoimmune disease, infections or cancer. Therefore, our goal is to elucidate the mechanistic connection between optimal affinity and efficacy to improve T-cell therapy.
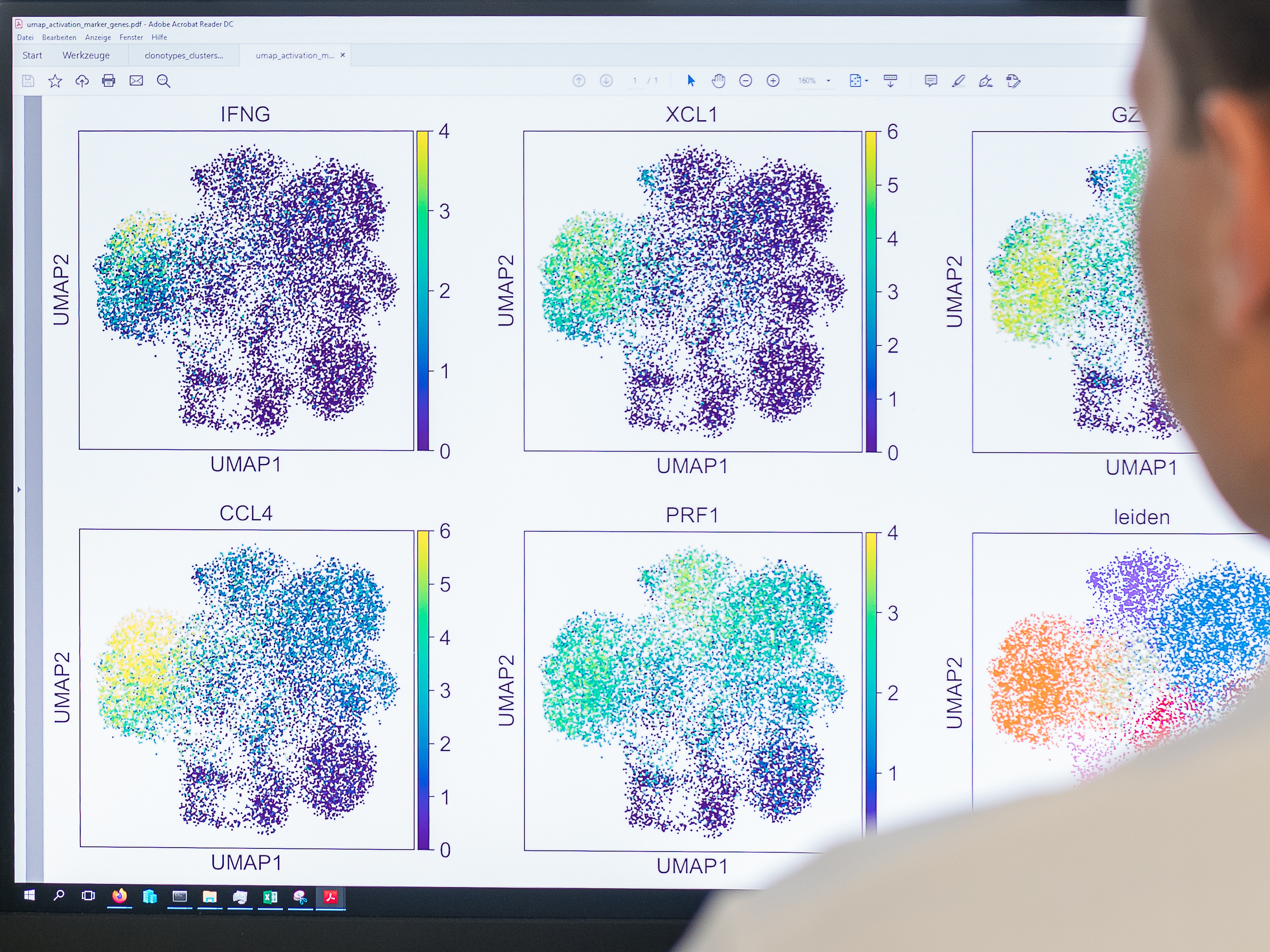
Light and shade of TCR
Adaptive T-cell responses arise from a polyclonal repertoire of naïve precursor cells. However, T-cell therapy in the context of TCR engineering usually comprises a single TCR for disease treatment. The combination of several T-cell clonotypes with different avidities and different landscapes of cross-reactivity against related targets provides flexibility in the immune response and can enhance protective capacity. Enabling polyclonality in immune responses is important in effective vaccination strategies.